Molecular Biomedicine | Multimodality molecular imaging of the alveolar-capillary barrier in lung disease using albumin based optical and PET tracers

Open the phone and scan
Inflammatory changes caused by viruses, bacteria, exposure to toxins, commonly used drugs and even surgical intervention have the potential of causing abnormal epithelial permeability, which is manifest as infiltrative processes on computed tomography (CT), including the widespread infiltrates seen in COVID-19 pneumonia and acute respiratory distress syndrome (ARDS). However, traditional imaging method (CT and plain chest radiography) can hardly detected early stage of these diseases. Herein, Prof. Akiva Mintz et al from Columbia University reported new approaches for multimodality molecular imaging of the alveolar-capillary barrier in lung disease using albumin based optical and PET tracers.
Viral pneumonia with associated ARDS has emerged as a significant public health threat during the COVID-19 epidemic, with a significant minority of diagnosed patients suffering from lung infection and hospitalization. While many patients who suffer COVID-related lung disease have comorbidities, it remains unpredictable which patients will suffer from lung complications until they demonstrate compromised oxygenation and abnormalities on plain chest radiography or CT. However, the presence of infiltrates indicates the late effect of the disease process, which is significant fluid in the lungs, and may not be a real-time measure of triaging early disease, quantifying the dynamics of the underlying alveolar and airway epithelial permeability, or an early predictor of response to treatment.
The researchers hypothesized that the real-time distribution of labeled albumin in the lungs is a feasible and informative method of directly evaluating the abnormal alveolar-capillary permeability that results in lung infiltrates. To demonstrated it, they evaluated the feasibility of 68Ga-albumin PET/CT to directly visualize the increased alveolar and airway epithelium permeability caused by inflammatory processes in the lungs.
Researchers utilized a previously published mouse model of ARDS, intranasal delivery of LPS, to induce the alveolar-capillary barrier permeability seen in lung disease. Mice were intravenously injected with Cy7 or 68-Gallium (68Ga) labeled mouse albumin and imaged using optical imaging (OI)/CT and PET. Significantly increased lung levels of Cy7-albumin on 3D OI/CT, which matched the abnormal appearance on microCT was observed (Fig. 1). This uptake correlated with fluorescence seen on sectioned lungs. To examine the translational potential of these findings, researchers radiolabeled albumin with 68Ga. It was found that in mice with LPS-induced lung injury, 68Ga-albumin PET correlated with the optical imaging findings and demonstrated abnormal activity in the lung fields, indicative of abnormal epithelial permeability. These findings indicate 68Ga-albumin can be utilized as a sensitive translational radiotracer for quantifying the abnormal epithelial permeability that is seen in various lung pathologies, including COVID-19 induced pneumonia and ARDS. The ability to use Cy7-albumin 3D OI/CT imaging as a preclinical translational surrogate for 68Ga-albumin offers an accessible high throughput means to rapidly screen potential therapeutics against lung diseases that clinically manifest with endothelial permeability.
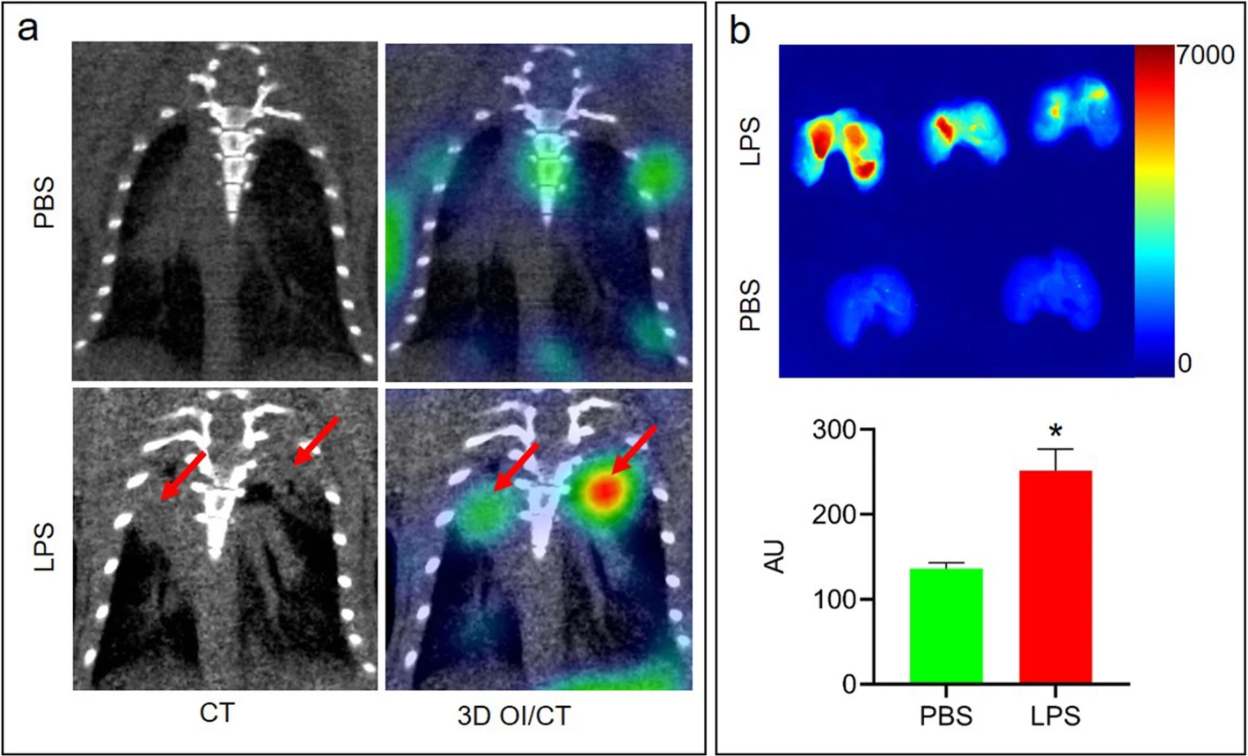
Fig. 1 3D OI/CT detection of LPS-induced lung inflammation using Cy7-albumin
Article Access: https://link.springer.com/article/10.1186/s43556-020-00019-8
Website for Molecular Biomedicine: https://www.springer.com/journal/43556
Looking forward to your contributions


