MedComm | Lipid metabolism in cancer progression and therapeutic strategies

Open the phone and scan
Dysregulated lipid metabolism represents an important metabolic alteration in cancer. Fatty acids, cholesterol, and phospholipid are the three most prevalent lipids that act as energy producers, signaling molecules, and source material for the biogenesis of cell membranes. The enhanced synthesis, storage, and uptake of lipids contribute to cancer progression. The rewiring of lipid metabolism in cancer has been linked to the activation of oncogenic signaling pathways and cross talk with the tumor microenvironment. The resulting activity favors the survival and proliferation of tumor cells in the harsh conditions within the tumor. Lipid metabolism also plays a vital role in tumor immunogenicity via effects on the function of the noncancer cells within the tumor microenvironment, especially immune-associated cells. Targeting altered lipid metabolism pathways has shown potential as a promising anticancer therapy. Here, the authors review recent evidence implicating the contribution of lipid metabolic reprogramming in cancer to cancer progression, and discuss the molecular mechanisms underlying lipid metabolism rewiring in cancer, and potential therapeutic strategies directed toward lipid metabolism in cancer. This review sheds new light to fully understanding of the role of lipid metabolic reprogramming in the context of cancer.

Cancer cells are characterized by aggressive proliferation. This requires that the cells develop strategies to acquire nutrients in a microenvironment that is deficient in O2 and general nutrient supplies. In addition to an increased demand for glucose, glutamine, and some amino acids, cancer cells undergo lipid metabolic reprograming that allows them to acquire the energy stores and membrane production required for rapid proliferation. A more comprehensive understanding of how cancer cells utilize lipid metabolic reprogramming to support their malignant phenotype may help identify novel therapeutic targets for cancer treatment (Fig 1). In cancer cell, Glutamine and glucose-derived acetyl-CoA are used in the de novo synthesis of cholesterol and fatty acids. In addition, exogeneous uptake also contributes to the fatty acid pool in cancer cells. Fatty acids can undergo β-oxidation to generate ATP and acetyl-CoA. FA-derived acyl-CoA and glucose-derived glyverol 3-phosphate serve the main material source for de novo synthesis of phospholipids.
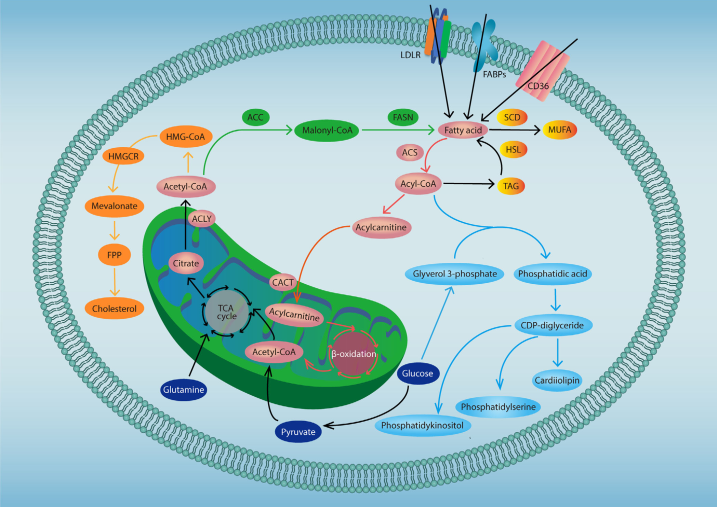
Fig. 1 Lipid metabolism in cancer
Article Access: https://onlinelibrary.wiley.com/doi/full/10.1002/mco2.27
Website for MedComm: https://onlinelibrary.wiley.com/journal/26882663
Looking forward to your contributions.


