Molecular Biomedicine | Developing inhaled protein therapeutics for lung diseases

Open the phone and scan
Biological drugs are revolutionising the treatment and management of many serious illnesses including cancer, autoimmune disorders, and rare genetic diseases, with about a third of all new drug approvals by the Food and Drug Administration (FDA) consisting of biological drugs. However, due to some challenges to develop inhaled protein biologic drugs, the development of inhaled therapeutics for the treatment of respiratory diseases has hardly been focused on inhaled protein biologic drugs over the past decades, with only one of which been approved by the FDA to date (Pulmozyme®). Here, Prof. Ruowen Ge et al. examines the important considerations and challenges in designing inhaled protein therapeutics for local lung delivery, and possiblle resolutions are also discussed.

Biologic therapeutics such as protein/polypeptide drugs are conventionally administered systemically via intravenous injection for the treatment of diseases including lung diseases, although this approach leads to low target site accumulation and the potential risk for systemic side effects. In comparison, topical delivery of protein drugs to the lung via inhalation is deemed to be a more effective approach for lung diseases, as proteins would directly reach the target in the lung while exhibiting poor diffusion into the systemic circulation, leading to higher lung drug retention and efficacy while minimising toxicity to other organs.
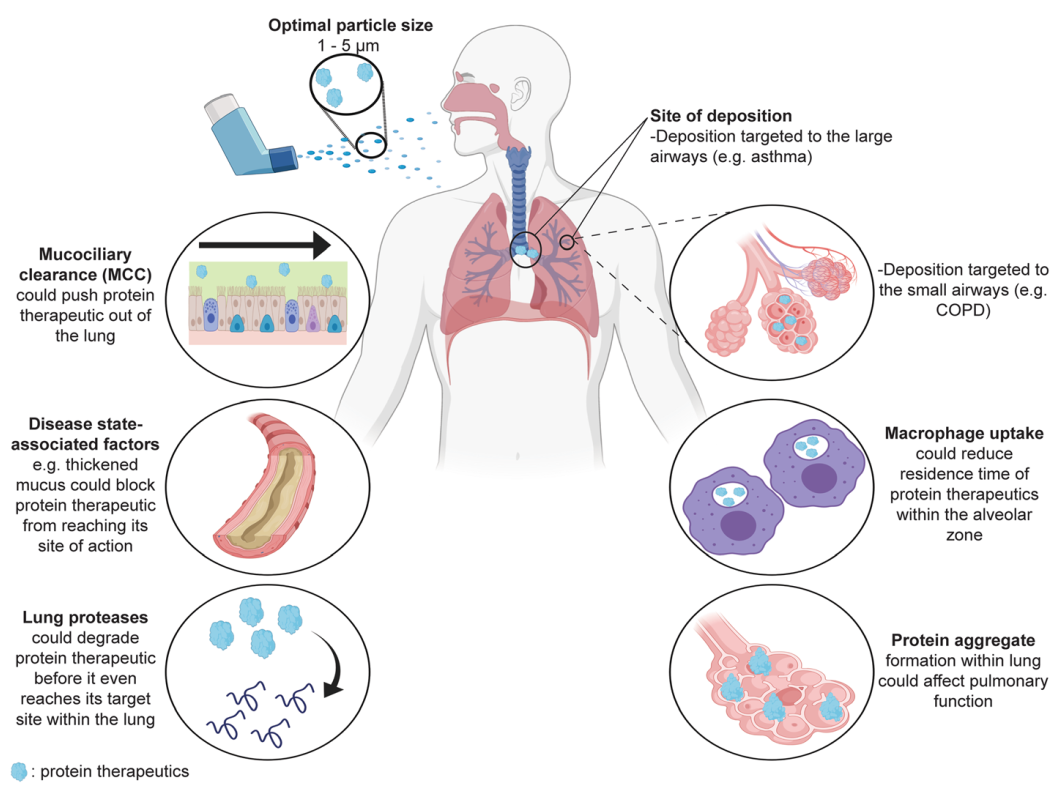
Fig. 1 Major challenges for targeting inhaled protein therapeutics to the lungs for local action
Article Access: https://link.springer.com/article/10.1186/s43556-020-00010-3
Website for Molecular Biomedicine: https://www.springer.com/journal/43556


