MedComm | Autophagic activation of IRF‐1 aggravates hepatic ischemia–reperfusion injury via JNK signaling

Open the phone and scan
Increasing evidence has accrued indicating that autophagy is associated with hepatic ischemia–reperfusion injury (IRI). This report demonstrates that interferon regulatory factor‐1 (IRF‐1) was upregulated in response to hepatic IRI and was associated with autophagic activation. As a result of these processes, there is an aggravation of liver damage, effects that can be offset by IRF‐1 depletion. In addition, these effects of IRF‐1 are associated with JNK pathway activation followed by increases in Beclin1 protein levels. This JNK‐induced autophagic cell death then leads to cell failure, and plays an important role in liver function damage. We conclude that IRF‐1 activates autophagy through JNK‐mediated autophagy. Accordingly, these findings indicating that the IRF‐1/JNK pathway activates autophagy to exacerbate liver IRI in this mouse model may provide new insights into novel protective therapies for hepatic IRI.
Hepatic ischemia–reperfusion injury (IRI) is a common condition associated with liver surgery and transplantation.Hepatic IRI exerts a critical effect on the outcome of liver surgeries and survival of these patients. Accordingly, it is not surprising that studies directed at exploring the molecular mechanism of liver IRI and the resultant progress of new protection strategy. However, hepatic IRI involves many factors and complex mechanism, making such investigations problematic.
One of the potential mechanisms associated with IRI is that of autophagy. Autophagy represents a critical process involving tightly regulated intracellular catabolism. As a result, it produces a degradation in cell subsets and lysosomes of proteins and participates in physiological and pathological processes. Interestingly, autophagy exerts a bidirectional regulatory effect with regard to its role in determining cell survival stage in response to IRI. Following environmental stress, autophagy can prevent the triggering of apoptosis in cells; however, under long‐term or severe external environmental stimulation, autophagy will lead to cell death. With regard to ischemia–reperfusion (IR), under conditions of moderate IR treatment, autophagy is upregulated to provide energy for cells, but with the application of stimulation beyond the tolerance of cells, high levels of autophagy were induced, leading to cell death. Autophagy is regulated by many molecular processes and signaling pathways, such as autophagy‐related gene (ATG), mTOR, PI3K, and AMPK. Recent findings from studies involving tumors, inflammation, and immune diseases have indicated that interferon regulatory factor‐1 (IRF‐1) can participate in the physiological function of cells by activating autophagy. Of particular relevance to the present investigation is the report that levels of IRF‐1 increase within the liver after IR exposure. Moreover, IRF‐1 knockout, which reduces the expression and release of high mobility group protein (HMGB1), relieves liver IRI.
Despite the potential significance regarding the molecular mechanisms of IRF‐1 in regulating autophagy in hepatic IRI, this area of investigation has received scant attention and therefore further study is sorely needed. In this report, authors examined whether the IRF‐1/JNK pathway activates autophagy and aggravates IRI in a mouse model of liver injury (Fig. 1). Such findings can serve as the foundation for the development of novel targets in the regulation of autophagy in hepatic IRI.
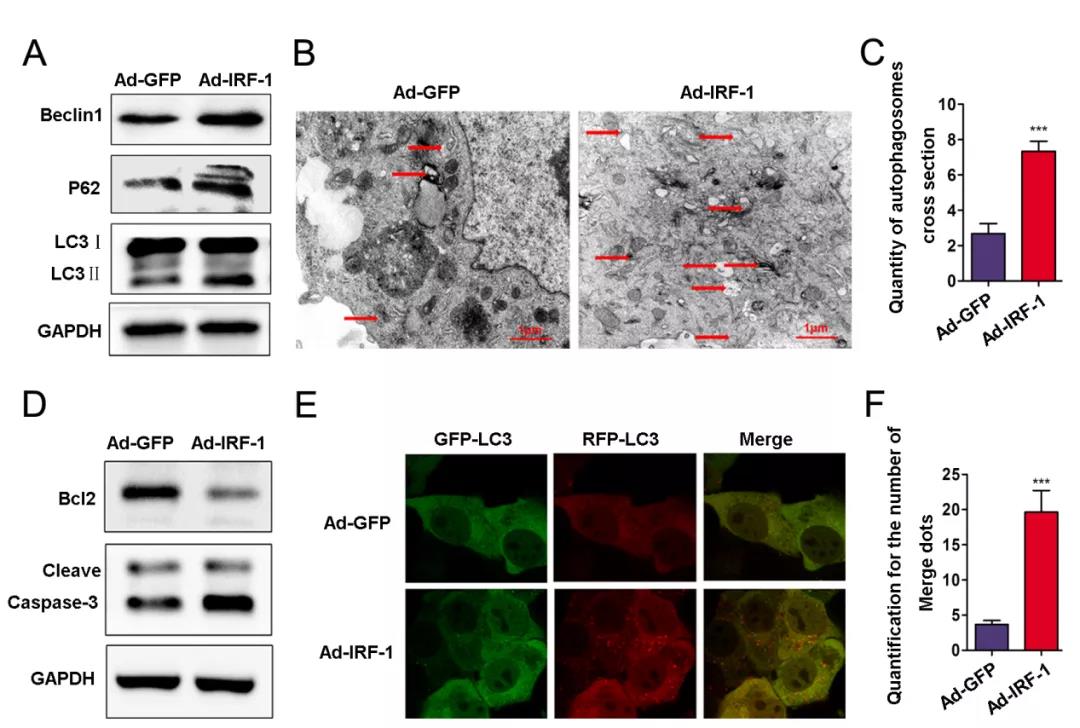
Fig.1 IRF‐1 overexpression increases autophagosomes in AML12 cells
Article Access: https://onlinelibrary.wiley.com/doi/10.1002/mco2.58
Website for MedComm: https://onlinelibrary.wiley.com/journal/26882663
Looking forward to your contributions.


