MedComm | Small extracellular vesicles‐based cell‐free strategies for therapy

Open the phone and scan
Small extracellular vesicles (sEVs) are extracellular nanovesicles that contain bioactive proteins, lipids, RNA, and DNA. A variety of biological process is regulated with sEVs. sEVs are an intercellular messenger regulating recipient cell function and play a role in disease initiation and progression. sEVs derived from certain cells, such as mesenchymal stem cells and immune cells, have the potential for clinical therapy as they possess the characteristics of their parental cells. With better understanding of sEVs biogenesis, their transportation properties, extended circulatory capability, and exceptional biocompatibility, sEVs emerge as a potential therapeutic tool in the clinic. Here, authors summarize applications of sEVs‐based therapies in different diseases and current knowledge about the strategies in bioengineered sEVs (Fig. 1), as well as the challenges for their use in clinical settings.
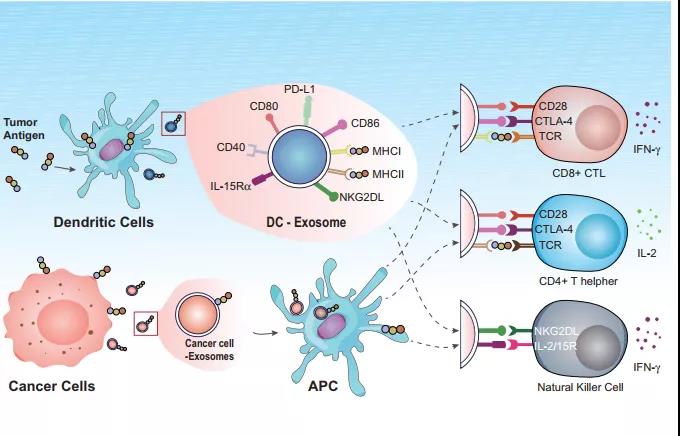
Fig.1 sEVs‐based vaccines in cancer therapy
Small extracellular vesicles (sEVs) are 30–200 nm lipid bilayer‐enclosed extracellular vesicles. A heterogenous mixture of sEV subsets, including exosomes, is derived when commonly used “exosome” isolation techniques are employed. Almost all cells generate sEVs and deliver to the surrounding biological fluids.Briefly, the formation of sEVs is initiated from endosomal system. Early endosomes mature into late endosomes or multivesicular bodies (MVB) by inward budding of the endosomal membrane. Constitutively, the intraluminal vesicles (ILVs) within large MVBs are formed during this process that the endosomal membrane invaginates. When fusion with the plasma membrane, most ILVs are released into the extracellular space, and turn to sEVs.
Small EVs were initially regarded as cellular garbage disposals as a result of cell damage, or by‐products of cell imbalance. Further studies found that those sEVs exhibited biological function. The bioactive cargo contains lots of information from their parental cell, including noncoding RNAs and mRNA, free fatty acids, surface receptors, and proteins inside and on the surface. Therefore, sEVs mediate the short‐range and distant communications between cells. Various target cells can be stimulated by the membrane molecules on sEVs or the contents inside sEVs. During tumor progression, sEVs from tumor cells may help to form a premetastatic niche for tumor metastasis.
Over the past decades, many studies demonstrated that sEVs were associated with various diseases, such as inflammatory diseases, diabetes, cardiovascular diseases, central nervous system diseases, tumors, and so on. Authors recently showed that PD‐L1 on sEVs may be used to predict melanoma patient response to anti‐PD1 therapy.
Article Access: https://onlinelibrary.wiley.com/doi/10.1002/mco2.57
Website for MedComm: https://onlinelibrary.wiley.com/journal/26882663
Looking forward to your contributions.


