MedComm | ENKUR recruits FBXW7 to ubiquitinate and degrade MYH9 and further suppress MYH9-induced deubiquitination of β-catenin to block gastric cancer metastasis

Open the phone and scan
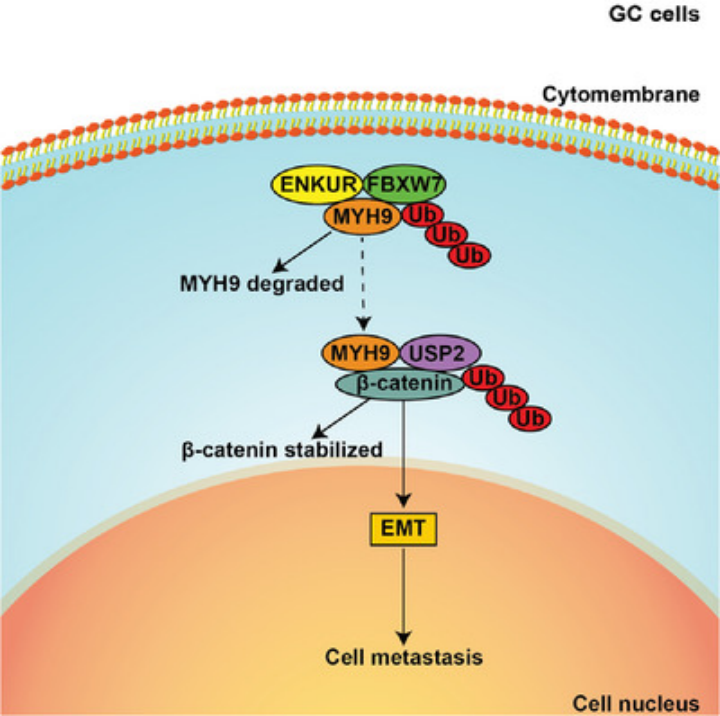
ENKUR inhibits MYH9 expression by recruiting the E3 ubiquitin ligase FBXW7 to promote its protein ubiquitinated degradation. Downregulated MYH9 further increased the degradation of β-catenin protein by reducing the recruitment of the deubiquitinase USP2 and finally decreased EMT signaling to inhibit GC cell metastasis.
Enkurin, TRPC Channel Interacting Protein (ENKUR) was shown as a suppressor in some tumors. However, the biological role of ENKUR on gastric cancer (GC) and its related molecular mechanisms is not clear. Here, we first observed that ENKUR significantly inhibited cell migration, invasion, and metastasis in GC. The molecular basis showed β-catenin-mediated epithelial-mesenchymal transition (EMT) signaling was inactivated in ENKUR-overexpressing GC cells. In addition, ENKUR knockdown markedly restored cell migration and invasion. Subsequently, ENKUR bound to MYH9 and decreased its protein expression by recruiting E3 ubiquitin ligase F-Box And WD Repeat Domain Containing 7 (FBXW7) to form an ubiquitinated degradation complex. The downregulated Myosin Heavy Chain 9 (MYH9) protein weakened the recruitment of the deubiquitinase USP2 and thus promoted the degradation of β-catenin protein, which finally suppressed EMT signaling. Finally, the oncogenic transcription factor c-Jun bound to ENKUR promoter and reduced its expression in GC. In clinical samples, decreased ENKUR expression promoted the unfavorable prognosis of GC. Our data proved the vital role of ENKUR on suppressing cell migration, invasion, and metastasis and demonstrated its potential as a therapeutic target for GC.
Article Access: https://doi.org/10.1002/mco2.185
More about MedComm: https://onlinelibrary.wiley.com/journal/26882663
Looking forward to your contributions.


