MedComm-Future Medicine | Direct cellular targets and anticancer mechanisms of the natural product oridonin

Open the phone and scan
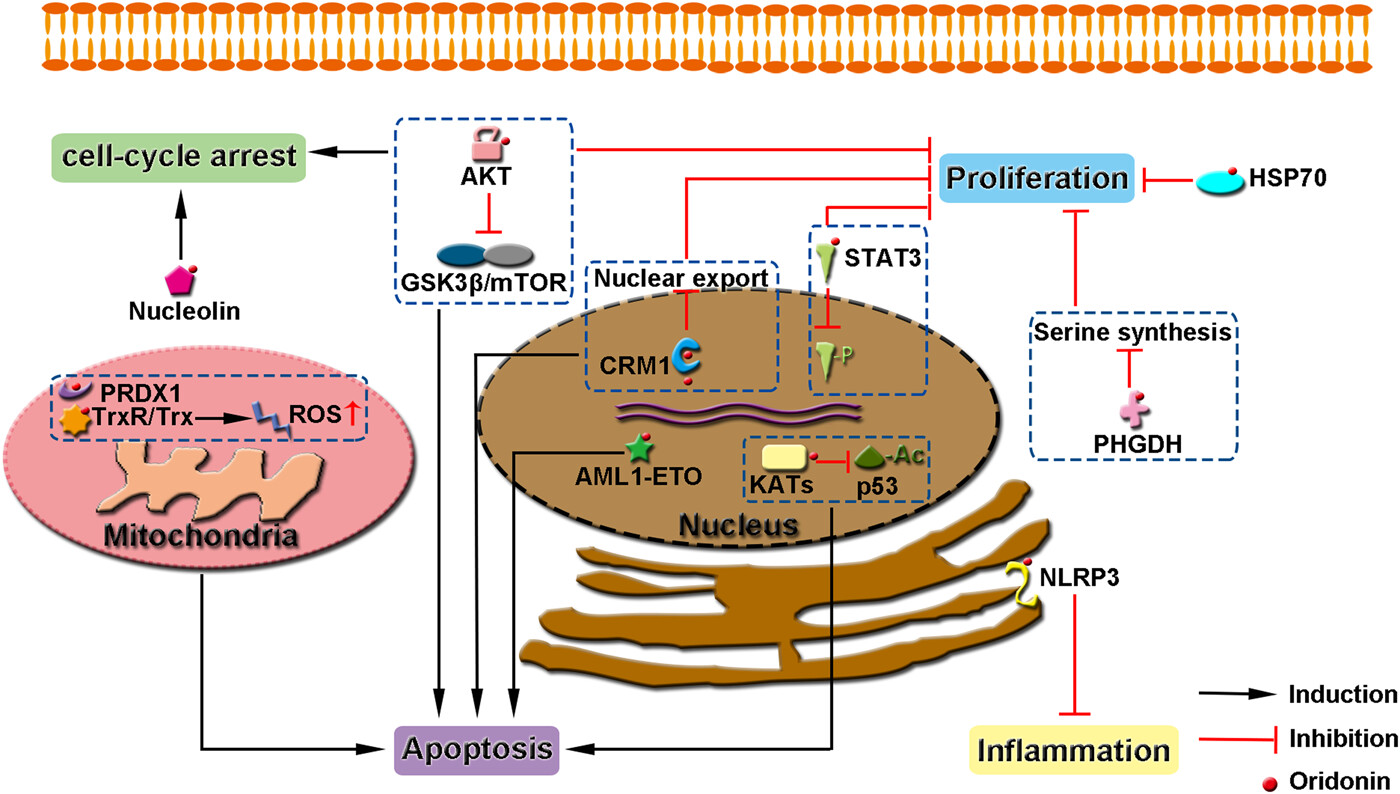
Direct cellular targets of oridonin and the functions of oridonin-binding proteins. For each protein and associated pathway, the extent of inhibition depends on the concentration of oridonin applied. Although these studies were generated using different cells, it is likely that these events occur simultaneously in all cells treated with oridonin (if the protein is expressed). Different cells may be more sensitive to different or different sets of oridonin-inhibited pathways.
Cancer is one of the leading causes of death worldwide. The proliferation, survival, and metastasis of cancer cells are governed by a complex series of intracellular and extracellular signaling pathways. Therefore, efficient cancer therapy often requires the modulation of multiple targets. Besides, the modulation of several biological targets with a single drug can lead to synergistic therapeutic effects and avoid side effects associated with combination therapy. Oridonin, an ent-kaurane type diterpenoid isolated from Rabdosia rubescens, has been reported to possess potent anti-inflammatory, antioxidant, anticancer, and neuroprotective activities. Among its many benefits, the anticancer effects have attracted the most attention because of its effectiveness in many tumor cells and its potential to overcome therapeutic resistance. Recently, several proteins involved in oncogenic signaling, including CRM1, PHGDH, HSP70, AML1-ETO, and STAT3, were identified as functional targets of oridonin and its analogs. The binding mechanism and the consequence of oridonin binding to these oncogenic proteins are summarized and discussed. These advances suggest that oridonin exhibits broad spectrum anticancer effects by targeting multiple oncogenic proteins mainly via Michael addition between its unsaturated ketone and the cysteines in the target proteins. Therefore, oridonin and its derivatives hold the potential to be developed as multitargeting anticancer drugs.
Article Access: https://doi.org/10.1002/mef2.35
More about MedComm-Future Medicine: https://onlinelibrary.wiley.com/journal/27696456
Looking forward to your contributions.


