MedComm-Future Medicine | Cordyceps sinensis ameliorates idiopathic pulmonary fibrosis in mice via inhibiting mitochondrion-mediated oxidative stress

Open the phone and scan
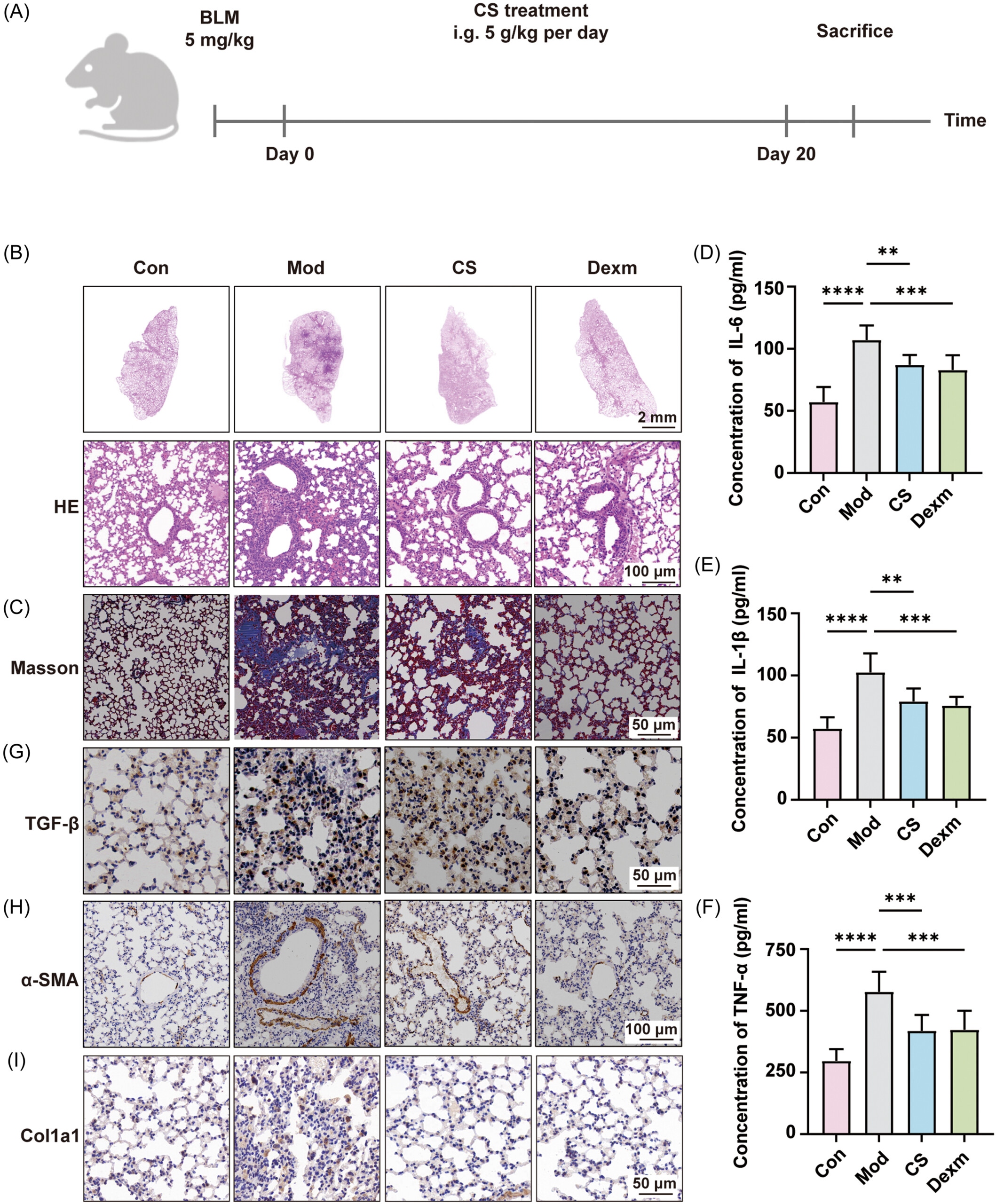
Cordyceps sinensis ameliorated bleomycin-induced idiopathic pulmonary fibrosis in mice. (A) Schematic representation of the model establishment and drug treatment regimen for pulmonary fibrosis in mice. Mice were divided into four groups: control group (Con), bleomycin-treated group (BLM, 5 mg/kg), dexamethasone-treated group (Dexm, 5 mg/kg), Bailing capsule-treated group (CS, 5 g/kg). (B, C) Representative images of lung tissue stained with hematoxylin and eosin and Masson's trichrome. (D–F) Levels of inflammatory cytokines interleukin-6 (IL-6, D), IL-1β (E), and tumor necrosis factor-α (TNF-α, F) in mouse serum. (G–I) Representative immunohistochemical images of transforming growth factor β (TGF-β, G), α-smooth muscle actin (α-SMA, H), and collagen type I alpha 1 (Col1a1, I) in lung tissues. Data are presented as mean ± SD (n = 5). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Idiopathic pulmonary fibrosis (IPF) represents a chronic interstitial lung disease with an unclear underlying mechanism and currently lacks a definitive treatment. Cordyceps sinensis (CS), renowned for its pharmacological properties in traditional Chinese medicine and extensive use in lung disease treatment, holds promise as a therapeutic agent for IPF. However, the specific role of CS in treating IPF remains unclear. In this study, we aimed to assess the efficacy of CS in treating IPF and unravel potential underlying mechanisms. Our results demonstrate that CS treatment effectively mitigated pulmonary inflammation and collagen deposition in bleomycin-induced IPF mice. Proteomics analysis revealed that the regulation of mitochondrial oxidative phosphorylation may serve as a potential protective mechanism of CS against IPF in mice. Further investigation unveiled that CS could suppress the excessive production of mitochondrial reactive oxygen species in lung tissues induced by bleomycin through moderating the expression and activity of mitochondrial complexes, thus safeguarding the integrity and function of mitochondria. Overall, our findings not only underscore the effectiveness of CS in preventing bleomycin-induced IPF but also highlight mitochondrial-mediated oxidative stress as a promising therapeutic target for treating IPF.
Article Access: https://doi.org/10.1002/mef2.91
More about MedComm-Future Medicine: https://onlinelibrary.wiley.com/journal/27696456
Looking forward to your contributions.


