MedComm-Future Medicine | Cell-type-specific mRNA m6A landscape and regulatory mechanisms underlying pulmonary injury in COVID-19

Open the phone and scan
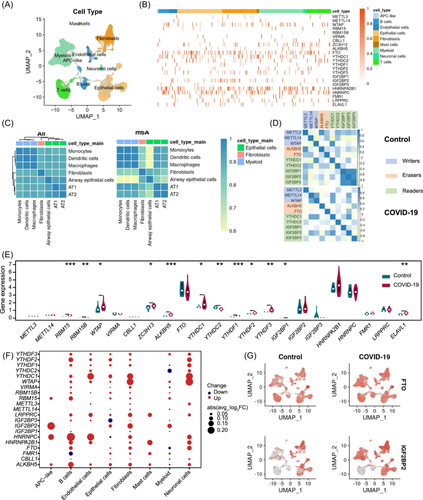
Expression profile of N6-methyladenosine (m6A) regulators. (A) Uniform manifold approximation and projection (UMAP) plot of 116,252 nuclei from control individuals and Coronavirus disease 2019 (COVID-19) patient samples. (B) Heatmap of each m6A regulators expression in each cell type. (C) Correlation clustering based on 2000 hypervariable genes (left), and m6A regulators (right). (D) Correlation analysis of the m6A regulators expression levels in control and patients with tissue samples as units. (E) Violin plot of the m6A regulators average expression levels. Control individuals were marked with blue; COVID-19 patients with red. (F) Differential expression of m6A regulators in different cell types of COVID-19 patients compared with control samples; The size of the dots indicates the average multiple of difference, and the color of the dots indicates upregulated (red) or downregulated (blue). (G) UMAP showed the expression of FTO and IGF2BP2 genes in control individuals and COVID-19 patients.
Coronavirus disease 2019 (COVID-19) pandemic has caused millions of deaths. The risk of COVID-19 spreading still exists after the deconfinement act, Omicron became the dominant variant. Although N6-methyladenosine (m6A) regulators has been reported to affect the pathogenicity of COVID-19, their mechanism in the progression of lung injury in COVID-19 patients remain elusive. Here we show the landscape and specific mechanisms of m6A regulators in lung tissues through single-nucleus RNA sequencing (snRNA-Seq) data sets of 116,252 cells, and the external validation was performed using data from another snRNA-Seq data. The m6A reader IGF2BP2 was specifically upregulated in alveolar type I (AT1) cells, resulting in impaired lung regeneration. ALKBH5 expression upregulation in macrophages, impairing immune responses. Moreover, WTAP markedly upregulated in fibroblasts, leading to pulmonary fibrosis. In addition, m6A regulators dysregulation induced aberrant cell–cell communication in pulmonary tissue and mediated ligand–receptor interactions across diverse cell types in lung tissues by activating the TGF-β signaling pathway. Overall, these results indicated that the upregulation of m6A regulators in alveolar cells, myeloid cells, and fibroblasts may induce pulmonary injury in patients. The development of m6A-regulator inhibitors could be as one potential antifibrotic drugs for COVID-19.
Article Access: https://doi.org/10.1002/mef2.94
More about MedComm-Future Medicine: https://onlinelibrary.wiley.com/journal/27696456
Looking forward to your contributions.


