Counties Suspend AstraZeneca Vaccine for Blood Clot Risk

Open the phone and scan
MedSci
MedSci is a leading medical research and academic service platform in China, committed to improving medical quality and providing intelligent and accurate decision support for clinical practice to benefit both doctors and patients.
Source: MedSci
Subscription Account of SCIMEA, your contributions are always welcomed here.
Introduction: Counties suspend AstraZeneca vaccine.
Irish health officials on Sunday recommended the temporary suspension of the AstraZeneca vaccine after reports of serious blood clotting after inoculations in Norway.
Dr. Ronan Glynn, Ireland’s Deputy Chief Medical Officer, said the recommendation was made after Norway’s medicines agency reported four cases of blood clotting in adults after receiving the AstraZeneca vaccine.
He said that while there was no conclusive link between the vaccine and the cases, Irish health officials are recommending the suspension of the vaccine’s rollout as a precaution. Denmark, Norway, Iceland, Italy, Romania and Thailand have also announced the delay of vaccination.
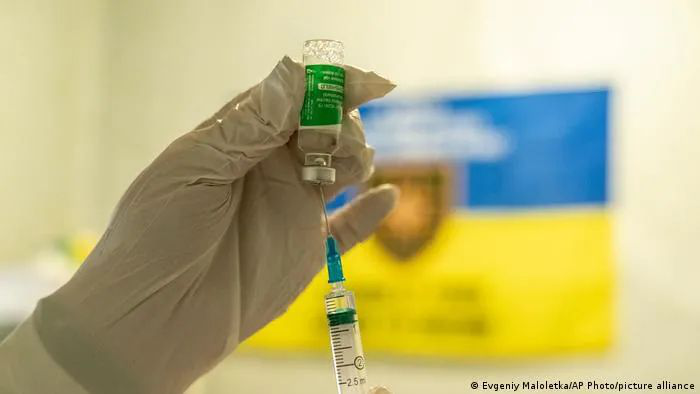
The Netherlands followed suit late Sunday, suspending vaccinations with the AstraZeneca shot as a precaution for two weeks. The health ministry said the move followed six new reports in Denmark and Norway of blood clotting and lowered levels of blood platelets in people aged under 50.
The Dutch medicines authority also stressed that no link has been proven between the cases and the vaccine. The health ministry said that no cases had been reported in the Netherlands.
AstraZeneca said in a statement Sunday that it “would like to offer its reassurance on the safety of its COVID-19 vaccine based on clear scientific evidence.”
“The safety of the public will always come first,” the British-Swedish biopharmaceutical company said, adding that it's “keeping this issue under close review but available evidence does not confirm that the vaccine is the cause.”
The company said that a review of safety data of more than 17 million people who have received the AstraZeneca vaccine in the European Union and the U.K. “has shown no evidence of an increased risk of pulmonary embolism, deep vein thrombosis (DVT) or thrombocytopenia, in any defined age group, gender, batch or in any particular country.”
Around 5 million Europeans have already received the AstraZeneca jab. There have been about 30 cases of "thromboembolic events" or developing blood clots
European Medicines Agency (EMA) said on Thursday (March 11) there was no evidence of an increased risk of clotting due to the vaccine, adding that “the benefits outweigh risks.”
The World Health Organization has previously said that there was no link between the jab and an increased risk of developing a clot.


