MedComm-Oncology | Next-generation sequencing identifies that protein tyrosine phosphatase receptor type D mutation is favorable to immunotherapy in human cancer

Open the phone and scan
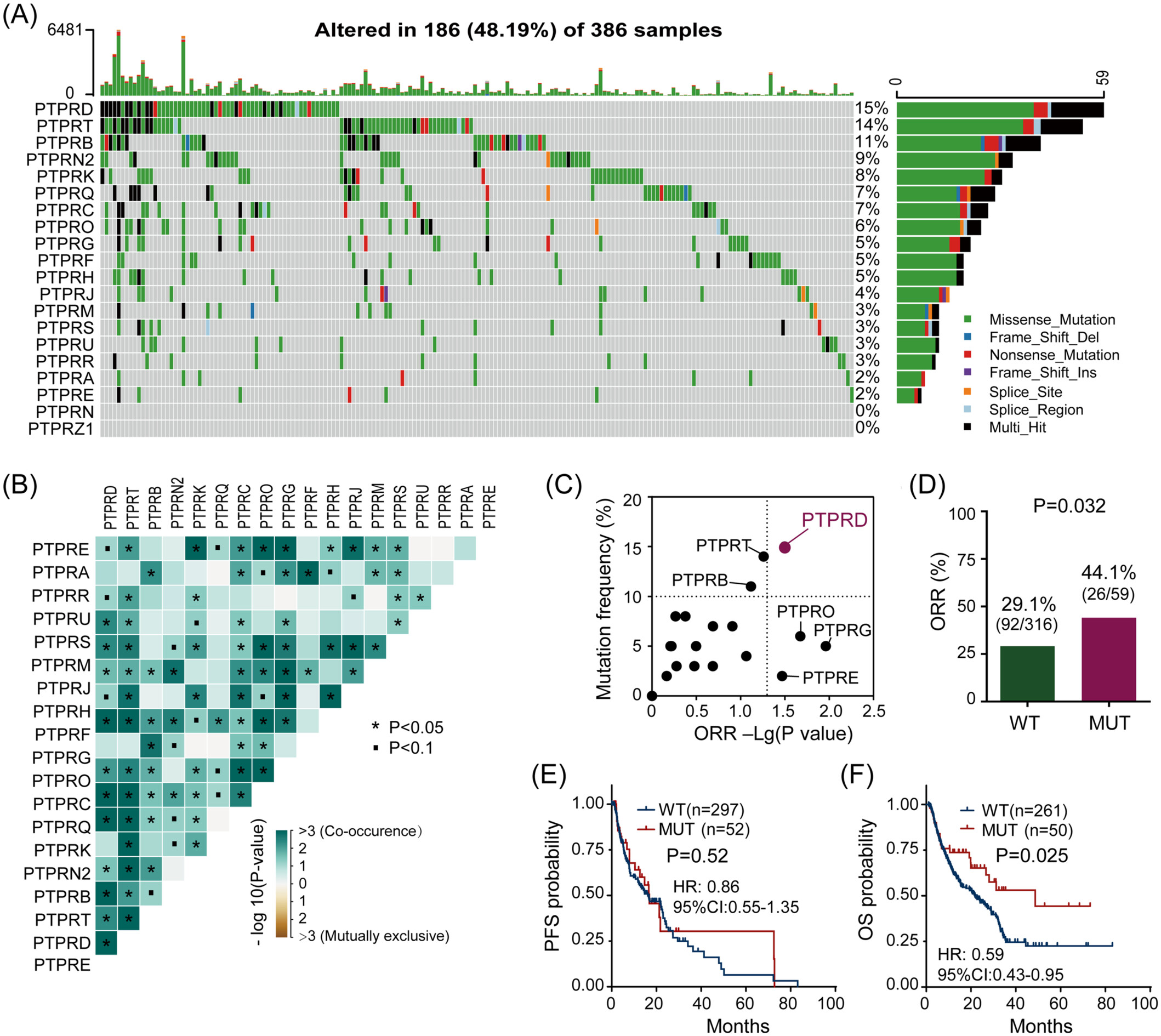
Protein tyrosine phosphatase receptor type D-mutation (PTPRD-MUT) was favorable to immunotherapy in the discovery cohort. (A) The genomic mutation signatures of PTPRs. (B) The co-occurrence and mutually exclusive among PTPRs. (C) Associations between objective response rate (ORR) and PTPR gene mutations. (D) The proportions of ORR in PTPRD-MUT and PTPRD-WT patients. (E, F) The progression-free survival and overall survival Kaplan–Meier curves in patients with/without PTPRD-MUT. HR, hazard ratio.
Protein tyrosine phosphatase receptors (PTPRs) play a crucial part in numerous tumor processes. However, the effect of PTPR mutations on the immune checkpoint inhibitor (ICI) response needs to be further clarified. Next-generation sequencing was performed on 453 cancer patients in our internal cohort. The genomic alterations, tumor mutation burden (TMB), neoantigens, and immune-related features/pathways of other cohorts were analyzed. Here, protein tyrosine phosphatase receptor type D (PTPRD) has a high mutation frequency and an intensified co-occurrence with other PTPRs. Patients who responded to ICI therapy were enriched with the PTPRD mutation (PTPRD-MUT). PTPRD-MUT patients had a higher objective response rate (44.1% vs. 29.1%), TMB/neoantigens, and longer overall survival time than PTPRD-wild-type (PTPRD-WT) patients. Genomic alterations with a higher mutation frequency of genes (such as LRP1B) were enriched in PTPRD-MUT patients. More abundant immune cells (including CD8+ T cells and macrophages) and upregulated immune-related genes were found in PTPRD-MUT patients. Moreover, Gene sets enrichment analyses showed that multiple antitumor immune pathways are activated in PTPRD-MUT patients. Therefore, PTPRD-MUT is beneficial for immunotherapy of multiple cancer types and may be a predictive biomarker of patient clinical outcomes.
Article Access: https://doi.org/10.1002/mog2.80
More about MedComm-Oncology: https://onlinelibrary.wiley.com/journal/27696448
Looking forward to your contributions.


