COVID-19 Vaccine against Omicron Developed by WestVac Biopharma Achieves Significant Progress

Open the phone and scan
According to the official website of WestVac Biopharma, in virtue of funding from the national emergency response project for the mutant COVID-19 vaccine, WestVac Biopharma Co., Ltd. (WestVac Biopharma) has achieved a significant progress in the second generation COVID-19 vaccine against the Omicron mutant strain.
The vaccine belongs to the latest fifth generation vaccine technology (fully synthetic vaccine), targeting the S-RBD protein of the COVID-19 mutant strain, and the subunit vaccine antigen is precisely designed based on the structure, which can self-assemble into stable nanoparticles. The large-scale production of vaccines adopts internationally advanced insect cell production technology and coordinates with international novel vaccine adjuvants. The vaccine can induce the production of high titers of neutralizing antibodies upon immunizing animals such as mice or monkeys. The neutralizing antibodies against the Omicron variant strain can reach a level of ten thousands (when blood is diluted 10,000 times, it still has the ability to block the virus from infecting cells). It was previously discovered that the vaccine has a level of ten thousands neutralizing antibodies that completely inhibits Brazilian strains, South African strains, Delta strains and other mutant strains of euvirus, suggesting that the vaccine is a universal COVID-19 vaccine against multiple mutant strains, and it is also the first COVID-19 vaccine announced internationally that has high titers of neutralizing antibodies against Omicron. Currently, the vaccine has completed process research and large-scale preparation. The production process is stable and it has been certified by the National Institutes for Food and Drug Control; the pharmacodynamic research on the immunogenicity and protection of mice, rats, and monkeys, as well as pre-clinical safety evaluation has been completed; the filing materials have been submitted successively to National Medical Products Administration in this August, it is expected to enter clinical trials in early 2022.
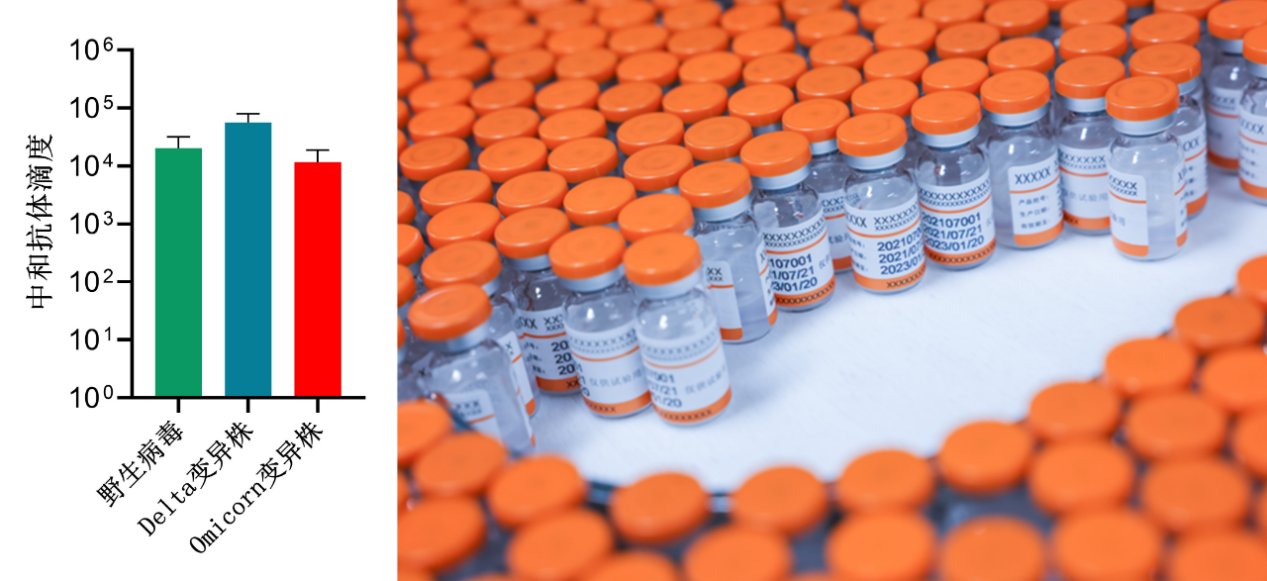
Neutralizing antibody titers of the second generation COVID-19 vaccine against wild viruses and different variants
Neutralizing antibody represents the ability of vaccines to induce antibodies to neutralize viruses, which is an important indicator to judge the effectiveness of the vaccine in the world. As per articles published in the latest Science journal, a number of clinical research data show that the neutralizing antibody titer directly determines the protective effect of the vaccine: If the neutralizing antibody titers NT50 induced by the vaccine are 10, 100, and 1000, the protective force upon vaccination is 78%, 91%, and 96%, respectively. WestVac Biopharma's second generation COVID-19 vaccine can reach a level of ten thousands neutralizing antibody NT50 against a variety of mutant strains. Therefore, it is reasonable to speculate that the vaccine is one of the most powerful weapons against various mutated strains of the COVID-19 virus.
WestVac Biopharma Co., Ltd. was jointly founded in July 2020 by 10 scientists from the State Key Laboratory of Biotherapy at West China Hospital of Sichuan University under the leadership of Academician Wei Yuquan, as well as the university, a team of scientists, and Chengdu High-Tech Industrial Development Zone. So far, the company has conducted Round A and B of financing, and has received funding of more than 2 billion yuan from a number of large financial investment institutions. The company has mature insect cell expression platform, mRNA vaccine platform, novel adjuvant platform, bacterial vaccine platform, tumor vaccine and immunotherapy platform, and has 21 pipelines including COVID-19 vaccine, multivalent influenza vaccine, herpes virus vaccine, and tumor immune preparations, etc. Among them, the Phase III clinical trials of the first generation COVID-19 vaccine has successfully completed enrollment, and clinical trials in Japan have been completed. The company has built a production base with an annual production capacity of 100 million doses in Tianfu International Bio-town in Chengdu High-Tech Industrial Development Zone, has completed the construction and verification of GMP production workshops, and obtained the “Drug Production License” issued by the SiChuan Medical Products Administration.
In terms of production, quality, and operation management, the company has introduced many professionals with rich experience in the vaccine and biopharmaceutical industries, established a complete production and quality management system, and has more than 300 employees to ensure the smooth large-scale production of vaccines. The production team includes many senior executives who have been trained in the GlaxoSmithKline system with more than 20 years of vaccine production experience, the quality system team has more than 20 years of experience in vaccine quality inspection, the marketing and sales teams include senior executives from international vaccine companies such as Pfizer and Sanofi Pasteur.



